

















Empower clinicians and their patients to make critical and timely healthcare decisions with the latest publications and clinical evidence
Trust is the cornerstone of doctor-patient relationships in the field of hereditary diseases. When a family has a child with a rare undiagnosed condition or a couple is planning their next chapter, they want assurance that their doctors are considering every peer-reviewed paper and all available evidence in their quest for an answer.
Connected to the world’s most thorough knowledgebase of expert-curated and up-to-date content, QCI for Hereditary Diseases ensures you leave no stone unturned in your search for a diagnosis.
QCI Hereditary Disease applications:

The phrase, “knowledge is power,” couldn’t be truer when it comes to diagnosing hereditary diseases.
With QCI for Hereditary Diseases, you can be confident that every clinical recommendation you make is backed by the latest peer-reviewed publications and vetted by M.D. and Ph.D.-level expert curators who do the reading for you.
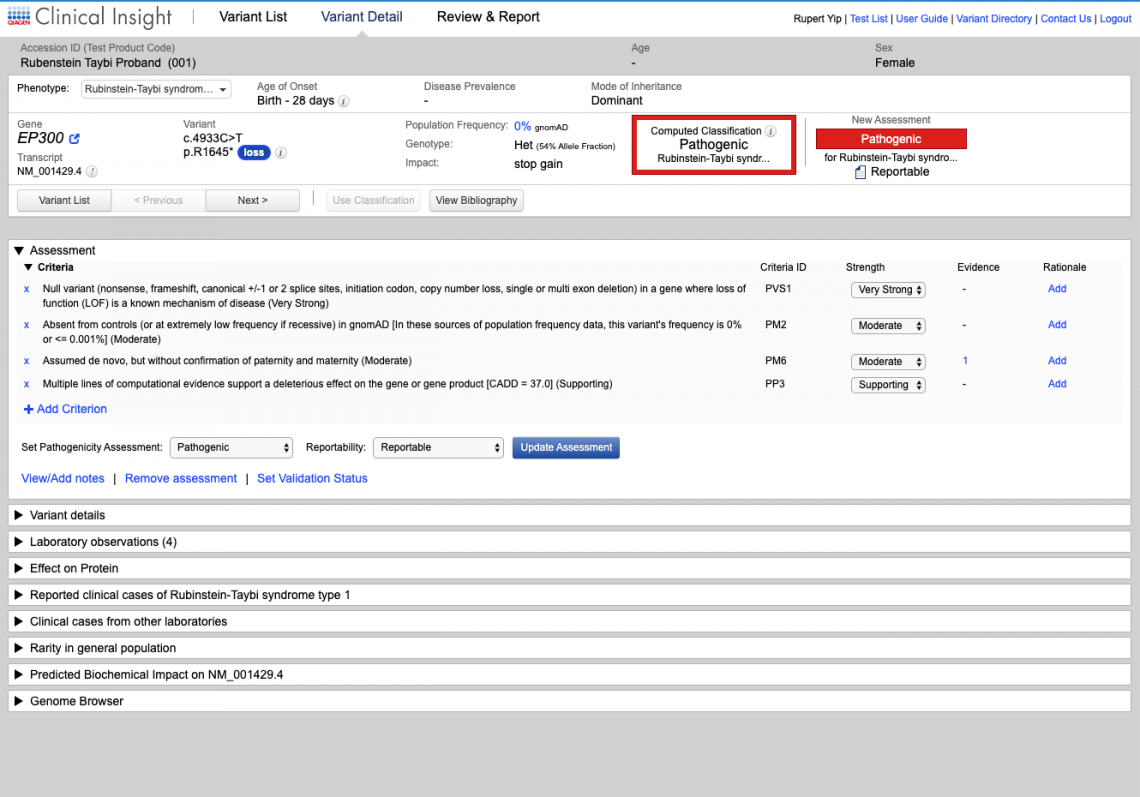
Unlike other clinical decision support tools and search engines, QCI for Hereditary Diseases delivers manually curated evidence directly to your pipeline. You receive links to all articles, auto-computed ACMG/AMP classifications, and access to over 1 million unpublished variant-phenotype relationships from the QIAGEN Knowledge Base.
Solve more cases faster, with data you can trust using the Human Gene Mutation Database (HGMD) Professional, the de facto standard resource for identifying inherited disease-causing mutations.

For molecular diagnostic labs performing variant interpretation and reporting in-house, QCI Interpret enables faster test turnaround times and higher confidence reporting for any assay on your sequencing platform.
From pipeline development to secondary analysis and variant curation, QIAGEN Clinical Testing Services offers customized end-to-end clinical reporting solutions to fast-track your lab’s scalability and profitability goals.

Whether new to NGS or an experienced user, learn how QCI for Hereditary Diseases can increase your lab’s efficiency, confidence, and test menu offerings.
Speak with a clinical testing expert today.